Our current work is about characterizing the adsorption diffusion, and desorption of simple molecules on and from amorphous water ice. We made a few improvements to our apparatus so we can work on very small number of molecules on ice surfaces (less than 1% of a layer) as appropriate to interstellar conditions. Furthermore we implemented a time-resolved scattering technique to obtain a better picture of kinetic processes of interaction of atoms and molecules with ice surfaces. Please go to the Publication tab to download our recent papers on diffusion, sticking and binding energies of simple molecules on water ice. Currently, our lab was moved to the Max Planck Institute of Astronomy, Heidelberg. At MPIA, the work utilizing our Syracuse University apparatus is led by Dr. Jiao He, Head of the Origin of Life Project. Jiao He was a graduate student and the a post-doc in our lab at Syracuse University.
Time of flight of an atomic oxygen beam and of a molecular oxygen beam to study chemical desorption
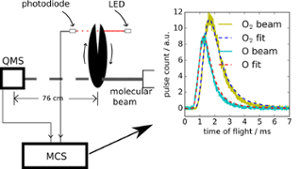 In this work, we measured the time of flight of O2, the product of reaction of H+O3(surface) –O2+OH. O2 desorbs due to the reaction and is detected in real time. He et al., ApJ 51, 104 (2017)
In this work, we measured the time of flight of O2, the product of reaction of H+O3(surface) –O2+OH. O2 desorbs due to the reaction and is detected in real time. He et al., ApJ 51, 104 (2017)
https://ui.adsabs.harvard.edu/#abs/2017ApJ…851..104H/abstract
Our recent research on oxygen on grains was highlighted in ScienceNews, follow the link:
http://news.sciencemag.org/chemistry/2015/05/why-there-so-little-breathable-oxygen-space
The paper reporting our experimental results and simulaitions was recently published in the Astrophysical Journal vol. 801, p. 120 (2015):
New determination of the binding energy of atomic oxygen on dust grains surfaces: experimental results and simulations
J.He, J.Shi, T.Hopkins, G.Vidali, (Syracuse University, Physics Department, Syracuse, NY) and M.Kaufman (San Jose’ State University, Physics Department, San Jose’, CA)
This research is supported by the Astronomy and Astrophysics Division of the National Science Foundation
Summary for the general public:
Oxygen is the third most abundant element in the Universe, but in the space between stars, the interstellar medium, it is not so easy to find it. In molecular clouds, where future stars will be born, molecular oxygen, O2, is found with abundance thousands of times below predictions or is not detected at all. To find out the abundance of atoms and molecules in space, data from astronomical observations are supplemented with data from laboratories that re-create the conditions in space. Both sets of data are then fed into computer simulations to predict the chemical composition for different regions of space. In the publication listed below, we report, using laboratory data and computer simulations, that an explanation for the “missing” oxygen can be ascribed to the fact that oxygen atoms are held on submicron dust grains (“interstellar dust”) much more tightly than previously estimated. Therefore, oxygen atoms are absent from the gas phase where they can make molecular oxygen, thus finding an explanation for the non-detection of molecular oxygen in many space environments.
See PDF of a an article in Today’s Science
Summary for the specialized reader:
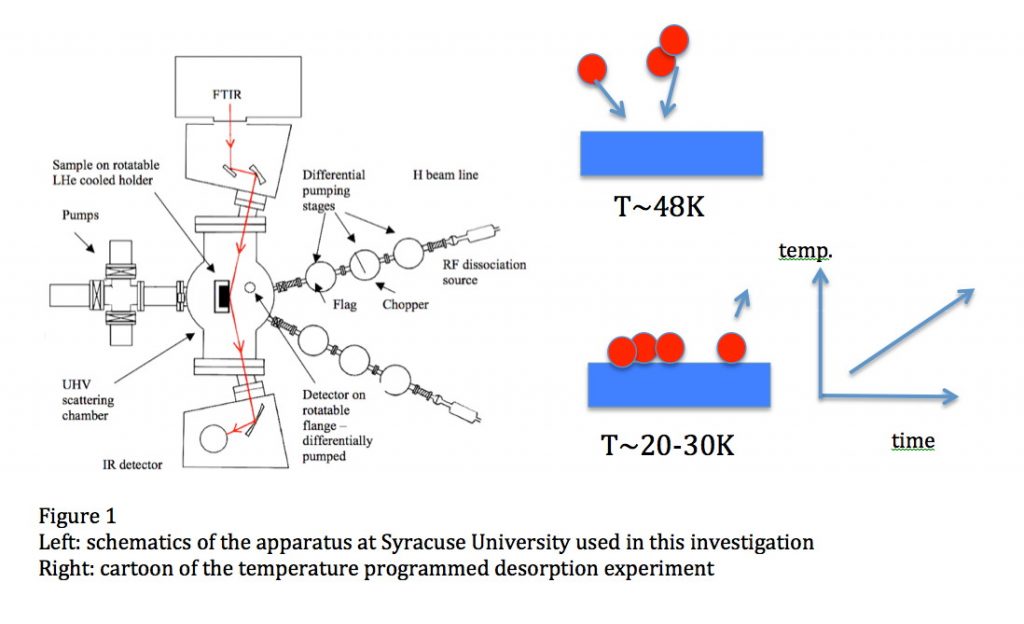
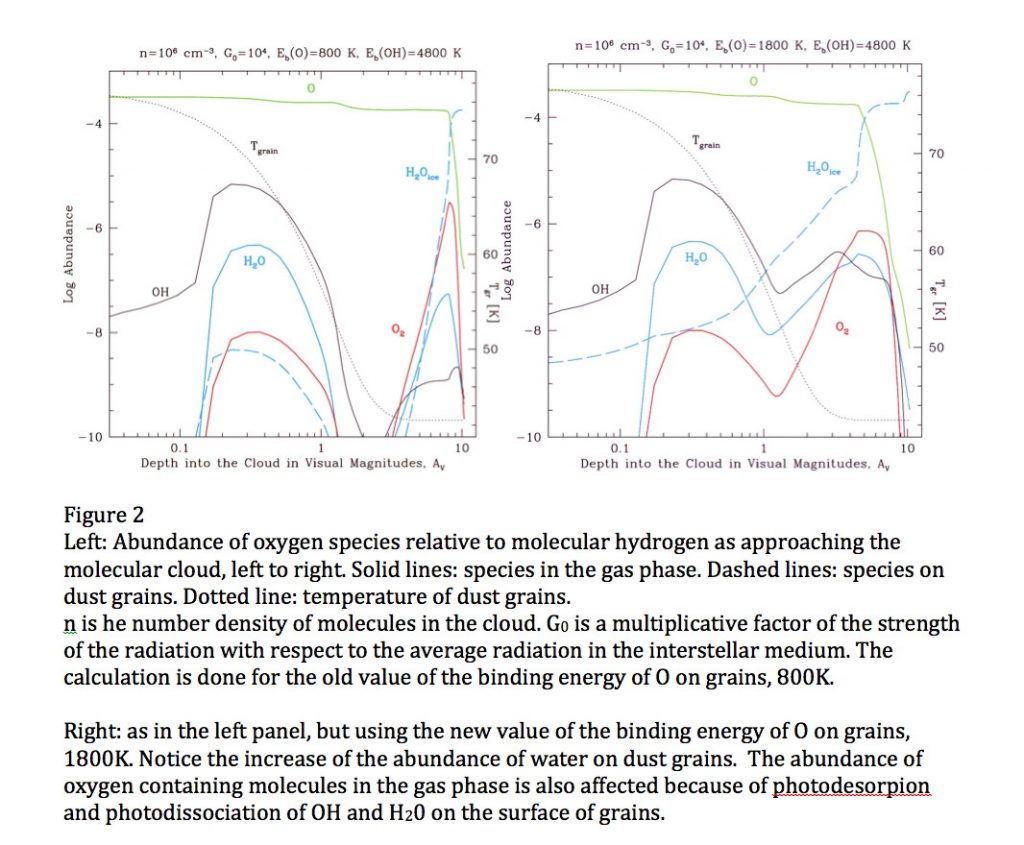
Oxygen is the third most abundant element in the Universe. In space, it is in submicron-sized silicate dust grains as well as in molecules, such as H2O, CO, CO2 and others, although not all the carriers of oxygen have been identified in all space environments. In cold molecular clouds, oxygen on dust grains is the main route to form water by subsequent reaction with hydrogen to form OH and then water, which are molecules of interest in astronomy and astrobiology. At the edge of these clouds, in the regions with intense far ultraviolet (FUV) radiation from stars, OH and water are photo-desorbed or dissociated, enriching the gas phase. The strength of the interaction between an oxygen atom and the surface of a dust grain (the binding energy) governs the time oxygen spends on the grain, and, consequently, its ability to diffuse and react. For a long time, the atomic oxygen-dust grain binding energy was estimated to be around 800K, but the non-detection of molecular oxygen in several space environments is puzzling and has led to the speculation that this binding energy should be much larger (Melnick et al., 2012). In a paper just published in the Astrophysical Journal – see above, we report the direct laboratory measurement of the energy needed to remove an oxygen atom from an amorphous silicate film mimicking an interstellar dust grain. The experiment consists of sending a beam of oxygen atoms (O2 is also present in the beam) onto an amorphous silicate film kept at the desired temperature in an ultra-high vacuum chamber, see figure 1, left panel. In a temperature programmed desorption experiment, the sample temperature is ramped and the oxygen atoms desorb and are detected, see figure 1, right panel. The temperature at which oxygen comes off is related to its binding to the surface. We find that this value is 1850K 90K and 1660K 60K for a bare and water-ice covered silicate, respectively, or about twice the previously estimated values. We then used such values in the simulation of the chemical evolution of an interstellar environment – a molecular cloud edge in star-forming regions in Orion exposed to FUV illumination, and found that OH and water formation on grains is considerably enhanced because of the wider temperature range in which oxygen can reside, see Figure 2, right panel. Because of the higher binding energy, gas-phase chemistry is controlled by the photodesorption or photodissociation of OH and H2O.
Bibliography
Melnick, G. J., Tolls, V., Goldsmith, P. F., et al. 2012, Herschel Search for O2 towards the Orion Bar, The Astrophysical Journal 752, 26